Describe the Process of Diffusion Using the Particle Theory
After all when liquid is poured from a glass so that it is empty it still contains air. Diffusion is the process by which particles of one substance spread out through the particles of another substance.
When chemicals like the smell of perfume or burning toast are let loose in a room the particles mix with the air particles.
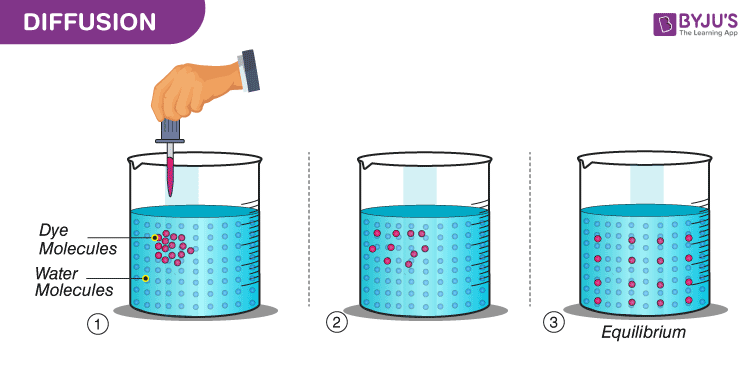
. Diffusion is the movement of particles from a high concentration to a lower concentration. Diffusion is a natural and physical process which happens on its own without stirring or shaking the solutions. 3 b Using the theory of kinetic energy of particle describe the process which the sugar cubes placed in the water.
Thus diffusion should not be confused with convection or dispersion which are other transport. A distinguishing feature of diffusion is that it results in mixing or mass transport without requiring bulk motion. That all particles are in constant motion.
In probability theory and statistics a diffusion process is a solution to a stochastic differential equation. 3 END OF PAPER. Use the picture and the particle theory to explain what happens to the particles in this solution.
The latter is a basic parameter directly analogous to a molecular diffusivity. The particles of smelly gas are free to move quickly in all. Solid liquid and gas.
By the kinetic molecular theory particle model all matter consists of particles there are spaces between the particles the particles are in constant random motion and there are forces of attraction and repulsion between the particles. The particles move in order to create equal concentration from higher to lower between all spaces of a place. Ideally according to this decision-making process a consumer passes from first knowledge of an innovation to forming a positive.
In the phenomenological approach diffusion is the movement of a substance from a region of high concentration to a region of low concentration without bulk motion. Scientists use models to explain things we cant see without advanced equipment. When a pure substances particle is moving from an area of high concentration to an area of lower concentration by occupying the spaces between the particles of another substance it is mixing or diffusing with.
D p k T A m p B k T. This is often described as a continuous view of matter. Diffusion is one of several transport processes that occur in nature.
Why is the volume of a sugar and water mixture less than the volume of each substance alone. One of these things is an. QUESTION 3 DIFFUSION OCCUR IN OUR DAILY LIFE.
Brownian motion is the random movement of microscopic particles suspended in a liquid or gas caused by collisions between these particles and the particles of the liquid or gas. The particle theory of matter states that all matter is made up of tiny particles specifically atoms and molecules and that these particles have inherent characteristics. Brownian motion reflected Brownian motion and OrnsteinUhlenbeck processes are examples of diffusion processes.
The most popular formula used in the literature about liquidsolid adsorption kinetics to describe diffusion-controlled processes is the intraparticle diffusion IPD equation. The molecules collide with each other and change the direction. Rogers 2003 diffusion of innovation theory for example describes the process by which an innovation disseminates through a societal group and focuses upon decision-making processes which lead to adoption of a new product or service.
Up to 24 cash back sugar particle colour particle water flavour particle This picture shows the mixed particles of a fruit-flavoured drink. Name and explain the process that caused this. Basically since the particles are in constant motion they move.
Diffusion is the movement of particles from a high concentration to a low concentration. Part icles of Your World 2015 by Crash Course Kids 349 min The Particle Theory of Matter is a scientific model. The particles of a substance gain energy and change from a regular ordered structure to a disordered structure with large distances between the particles.
Three states of matter are. A major part of the theory is the belief that all particles in a single pure substance are the same and are different from particles of other substances. A sample path of a diffusion process models the trajectory of a particle.
The particles of both the substances will mix or diffuse faster when the temperature is high. The particle theory states that there are spaces between all particles. The theory also.
Diffusion follows a basic idea in the particle theory. Even when children are introduced to the idea of matter being made of particles most consider there is still stuff between the particles and that something like air fills the empty space. Using the theory of Brownian motion Einstein derived the relationship for particle diffusivity.
This means that in a glass of water there are many water particles but also many empty spaces. Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. The rate of diffusion depends on the gradient in particle concentration and the particle diffusivity.
It can be seen as a spreading-out of particles resulting in their even distribution. According to Ficks laws the diffusion flux is proportional to the negative gradient of concentrations. Furthermore temperature is defined to be a measure of the average kinetic energy of the particles.
A scientific model is a way of illustrating ideas objects and processes so theyre easier to understand. This process is called diffusion. A Define the process diffusion.
Placing a drop of food coloring in water provides a visual representation of this process the color slowly spreads out through the water. It is a continuous-time Markov process with almost surely continuous sample paths. It goes from regions of higher concentration to regions of lower concentration.
Using particle theory imagine that you have a substance ie perfume. Diffusion is how smells spread out. When you spray the perfume at first.
Evaporation is a change of phase. Liquid and gases undergo diffusion as the molecules are able to move randomly. After a time the water tastes sweet.

Diffusion Worksheet Worksheets Term Paper Research Paper
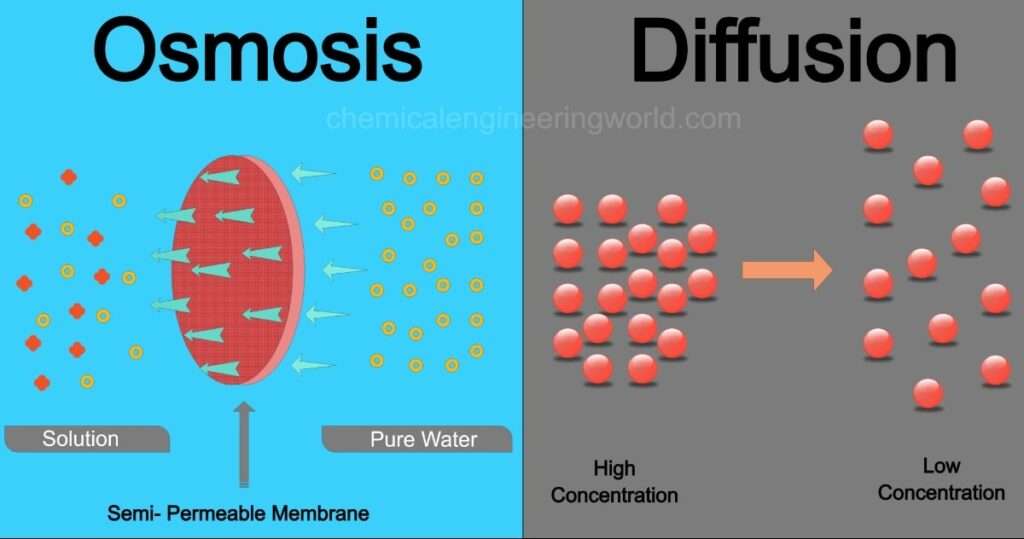
Difference Between Osmosis And Diffusion Chemical Engineering World

What Is Diffusion Definition Types Examples Of Diffusion

Diffusion Of Particle An Overview Sciencedirect Topics

Passive And Active Transport Worksheets Passive Transport Biology Worksheet Cells Worksheet

Our Progression Charts Provide Clear Guidelines To Support You In Assessing Your Students Progress At Ks3 Science Dow Secondary Science Progress Book Science

Diffusion In Solids Liquids And Gases Geeksforgeeks

Educational A1 Poster Particles Of Matter Poster States Of Matter
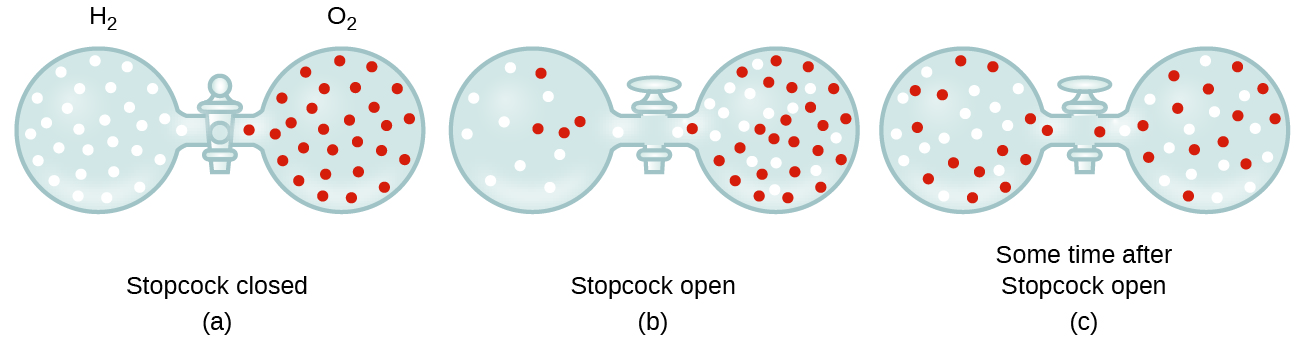
9 4 Effusion And Diffusion Of Gases Chemistry

Diffusion Of Gases Properties Of Matter Chemistry Fuseschool Youtube
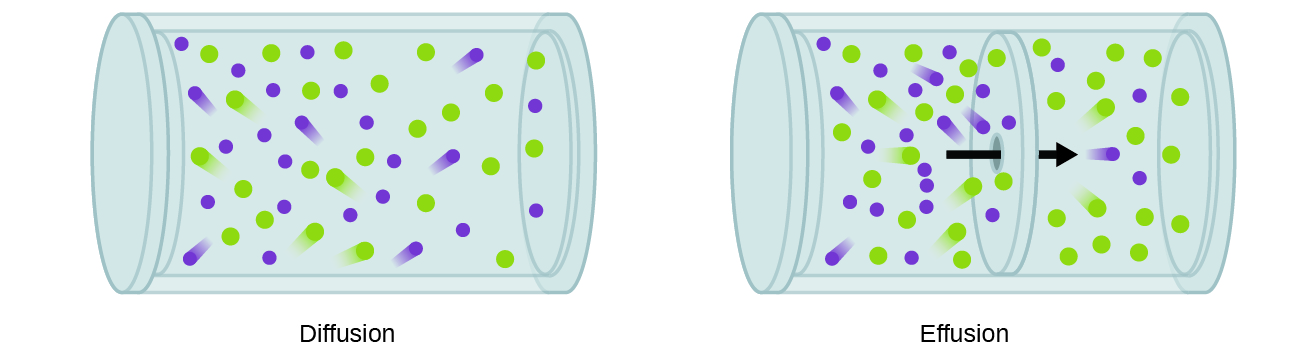
9 4 Effusion And Diffusion Of Gases Chemistry
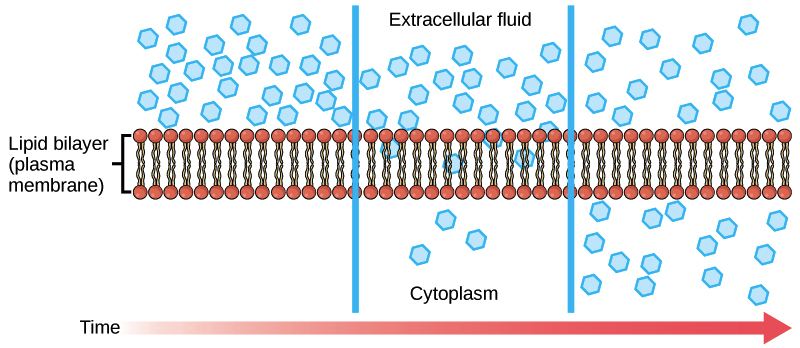
Simple Diffusion And Passive Transport Article Khan Academy

Image Result For Cell Membrane Worksheet Cell Transport Cell Membrane Biology Units

Diffusion Overview Chemistry What Is Diffusion Video Lesson Transcript Study Com

Gas Diffusion And Effusion Introduction To Chemistry

Diffusion Introduction Video Khan Academy

Diffusion Overview Chemistry What Is Diffusion Video Lesson Transcript Study Com


Comments
Post a Comment